FDA orphan drug designation for Thin Endometrium.
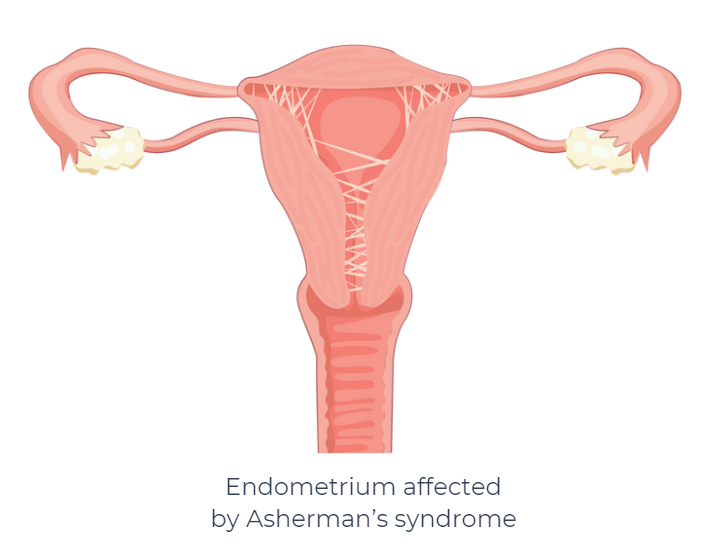
Asherman syndrome (AS) is an uncommon gynecological disorder that occurs when scar tissue (intrauterine adhesions) forms inside the uterus, often after surgery such as dilation and curettage (D&C), a cesarean section, or uterine infection.
These adhesions can lead to symptoms like:
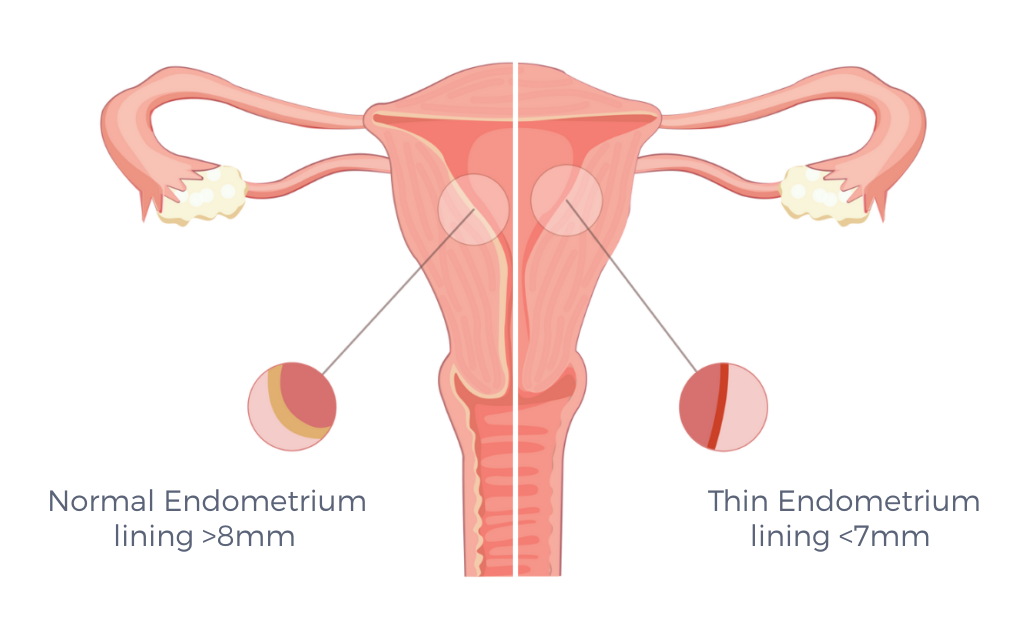
Thin Endometrium (TE) is another rare condition in which the endometrium is too thin to support implantation or a healthy pregnancy. It is often caused by:
ENDORENEW has developed PAULA, a new cell-based therapy using human autologous CD133+ bone marrow-derived stem cells (BMDSCs) for endometrial regeneration, aimed at restoring normal endometrial function in patients with moderate to severe Asherman syndrome or thin endometrium.
The PAULA therapy process includes these steps:
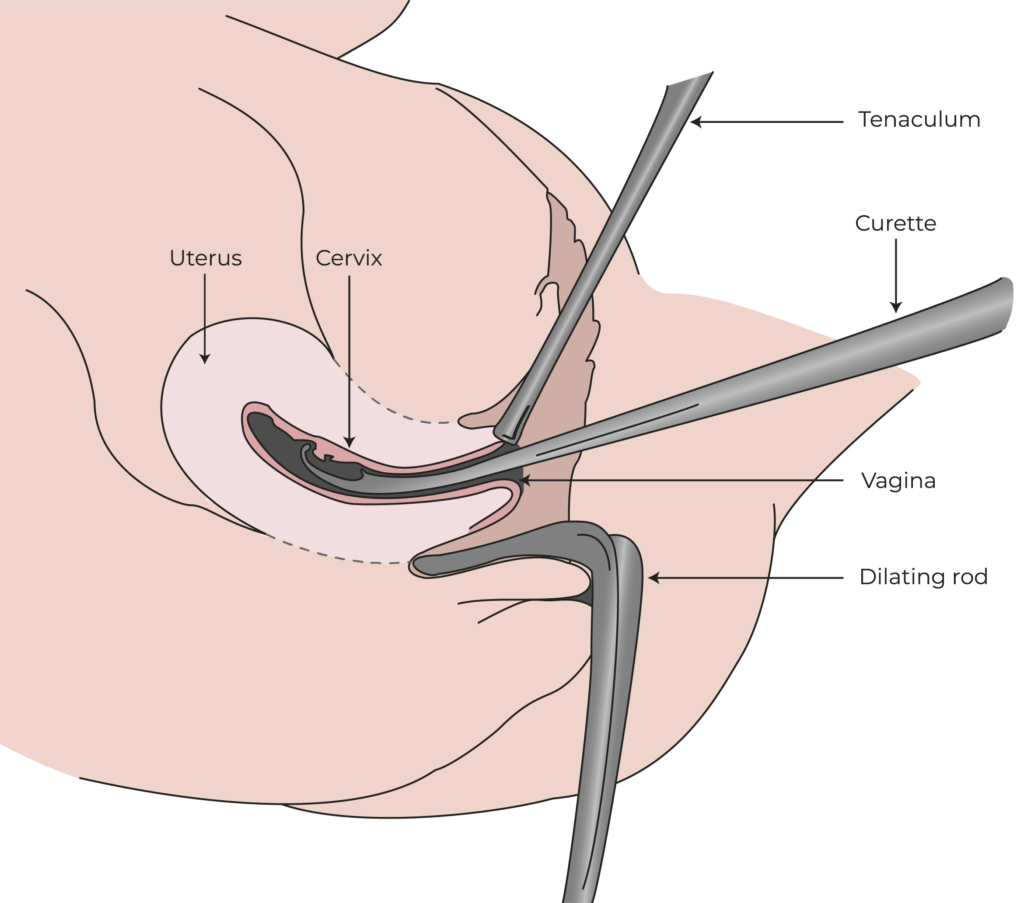
Initial diagnostic hysteroscopy to confirm eligibility for therapy.
Administration of granulocyte colony-stimulating factor (G-CSF) to mobilize CD133+ stem cells into the bloodstream.
Apheresis to collect the mobilized CD133+ stem cells from the blood.
Preparation and enrichment of the stem cells in a Good Manufacturing Practice (GMP)-certified lab.
Infusion of the enriched stem cells into the uterine arteries via catheterization to promote endometrial regeneration.
Phase I/II treated 20 patients showing increased endometrial thickness, improved uterine cavity conditions, restored regular menstrual cycles, and successful pregnancies. Endorenew will conduct a Phase III trial planned for mid-2026 in Europe to consolidate these findings.
PAULA has been tested in clinical trials with promising results. The therapy is safe and effective, with patients experiencing minimal side effects. The use of autologous stem cells reduces the risk of immune reactions, ensuring compatibility and improving overall therapy outcomes.
PAULA has shown proven results in clinical trials, demonstrating significant improvements in endometrial thickness, restoration of menstrual function, and positive fertility outcomes. The therapy is well-tolerated with no major adverse effects reported.
Traditional treatments for Asherman syndrome and thin endometrium often rely on surgical adhesiolysis and hormonal therapies that provide temporary relief but do not regenerate the uterine lining.
PAULA therapy takes a regenerative approach by using autologous stem cells to restore the endometrial tissue. This unique therapy promotes angiogenesis (new blood vessel formation) and cellular repair, offering a long-term solution to improve endometrial thickness and fertility outcomes.